PRODUCT – Allergen
Open a new path for allergy diagnosis and therapy
House Dust Mite, HDM
HDM
House dust mites (HDM) are the most common cause of allergies, and it is known that more than 70% of allergic rhinitis patients are caused by house dust mite.
Prolagen Co., Ltd. has various types of diagnostic and therapeutic allergens using house dust mite mass-reared using its own technology.
Product information
| Class | Cat No. | Product Name | Unit | Etc. |
| Mite | PEA-DERF010 | Mite, House Dust Dermatophagoides. farinae (DF) | 10mg protein / vial | KFDA Approval Code 201908453 |
| PEA-DERP010 | Mite, House Dust Dermatophagoides. pteronyssinus (DP) | 10mg protein / vial | KFDA Approval Code 202401780 | |
| PEA-TYRP010 | Mite, Storage Tyrophagus putrescentiae (TP) | 10mg protein / vial |
Features
- Obtained KFDA pharmaceutical product approval (America, Europe house dust mite)
- Manufactured through strict pharmaceutical manufacturing process and quality testing
(Korea’s only allergen pharmaceutical manufacturing GMP) - Freeze-dried formulation provides good stability and is easy to apply to various formulations.
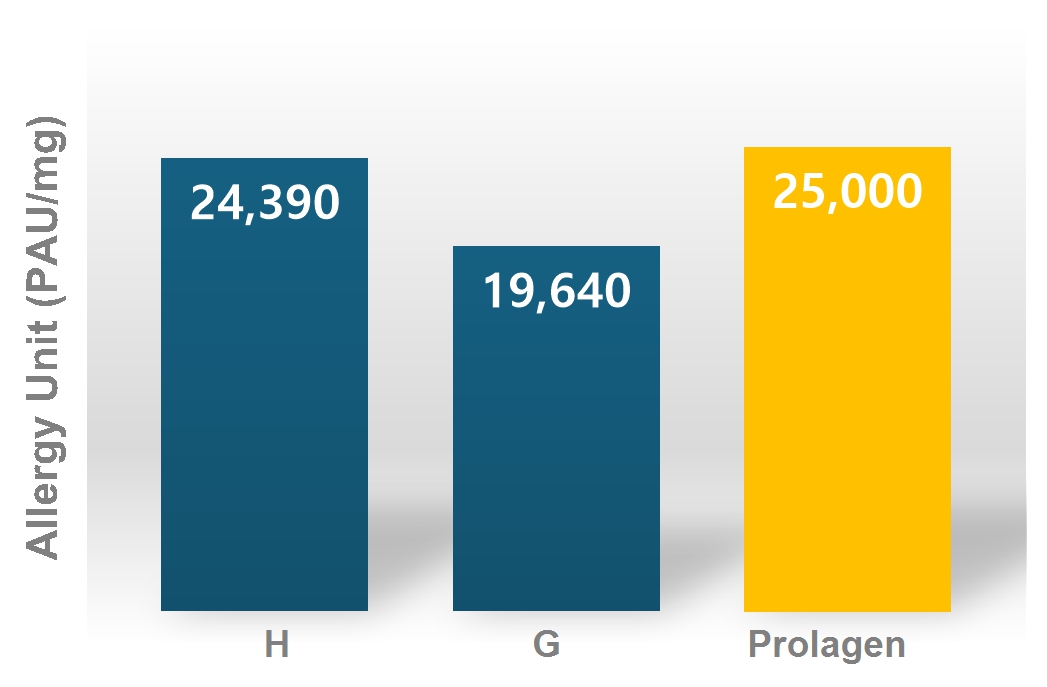
- Equivalent or higher AU (Allergy Unit) compared to overseas products, application cases in more than 35 papers

▶ Der. farinae Allergen Potency comparison
▶ Reference